Cervical Cancer
Introduction
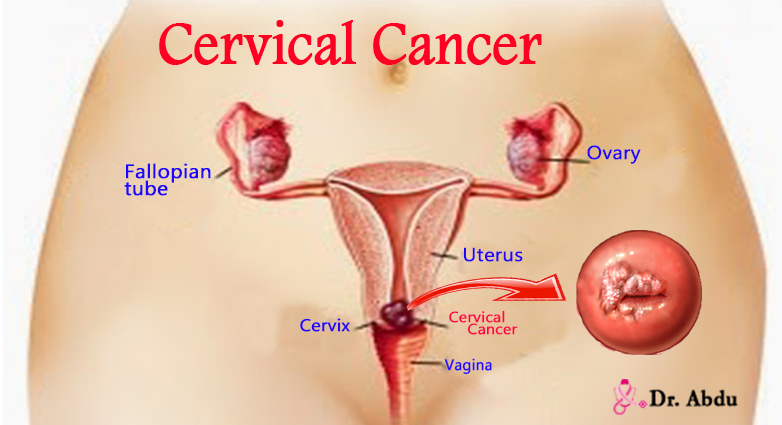
Cervical cancer, a largely preventable disease, is the most common genital tract malignancy in the female and is a major public health problem in low- and middle-income countries (LMICs) (1). It occurs when the cells of the cervix grow in uncontrollable manner and invade other surrounding tissues (bladder, vagina, and rectum) and distant organs of the body (commonly the lungs, liver) (2-3) (figure 1).

Cervical cancer starts from the cervix — the lower part of the uterus that connects to the vagina (3).
Cervical cancer is slow-growing cancer, so its progression through precancerous changes provides opportunities for prevention, early detection, and treatment (2). A striking reduction has been shown in the incidence and mortality from cervical cancer the past century in countries which were able to establish successful national screening programs. These programs relied on cytology-based Papanicolaou smears to identify cervical cancer precursors that can be removed before progressing to invasive cancer. Prevention of up to 91% of all invasive cervical cancers has been achieved in countries able to implement widespread cytology-based screening. However, these cytology-based programs are expensive and require a robust and well-funded health care system which is difficult to have in most LMICs. The unequal burden of cervical cancer is an example of the impact of unequal access to health care. Fortunately, alternative strategies to prevent cervical cancer have been investigated and extensively evaluated in these settings in recent years. The recent introduction of two commercially available vaccines against human papillomavirus (HPV) has also offered the possibility of primary prevention of cervical cancer.
Burden of the Disease (BOD)
According to globocan 2018 report cervical cancer is the second leading frequent cancer and cancer related death following breast cancer globally. There were a total of 569,847 new cases, with 311, 365(54.6%) mortality in 2018 alone (5).Globally, incidence and mortality rate have been declining in most areas of the world in the past 30 years (1.6% per year) due to increased access to health services, reductions in some risk factors (such as fertility rates), improvements in treatment, and successful cytology-based screening program. In contrast, in LMICs, the number of new cases and deaths has increased constantly by about 0.5% per year because of population aging, poor screening facilities and treatment. This resulted in the fact that more than 80% of cases and 88% of deaths occur in LMICs (1). In high-income countries (HICs), generally, rates are lower (5/100,000 women). However, incidence and mortality rates are highest among the poorest or most marginalized women reflecting the variability in accessibility of services.In Latin America and the Caribbean, Guyana, Honduras, Jamaica, and Nicaragua have rates around 40/100,000. In Asia, the highest rates are in Bangladesh, Cambodia, India, and Nepal. Cultural and religious practices that influence and govern sexual behavior and transmission of HPV has a huge impact on cervical cancer. Sub-Saharan Africa has the highest estimated rates of cervical cancer; in Guinea, Malawi, and Zambia, the age-standardized incidence rate is over 50/100,000. On the other hand, in Middle East countries and North Africa, such as Algeria, Egypt, Libya, Sudan, and Tunisia, where sexual behaviors are more conservative, the recorded incidence rates are below 10/100,000 (1,5-6).
Despite the absence of nation wide population based cancer registry, according to the Addis Ababa Cancer Registry (AACR) report on the incidence data based on the first two years of registration (2012–2013), there were 4139 newly diagnosed patients the majority of which (67%) were females. Cancers of the breast (31.5%) and cervix (14.1%) were the two most common cancers among females (figure 2) (7).
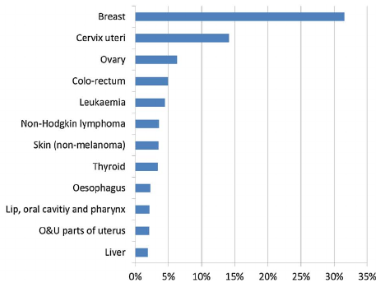
One of the most notable characteristic of cervical cancer is that it affects relatively young women; the burden of disease among women under age 40 years is high compared with other cancers, because of the large numbers of women in these age groups in LMICs and the fact that cervical cancer rates begin to rise at younger ages than other cancers (1). In the above report, females of age group 30–49years comprises for 40.8% cases (7).
Risk Factors
Though a total of 66 HPV types infect the genital mucosa, persistent infection with high-risk types of HPV (HPV 16 and 18) which have been detected in ~70% of all cervical cancer cases worldwide is considered as the necessary cause. Moreover, age and lifetime number of sexual partners are significant risk factors (1, 8-9).
Other risk factors, especially common in sub-Saharan countries, include HIV/AIDS, early age at marriage and first sexual intercourse (20 years old), multiple sexual partners, tobacco smoking, oral contraceptive pill use for more than 5 years, low socioeconomic status, history of cervical cancer in the family, high parity (more than 3 children born), and immune-depression due to malnutrition or other systemic diseases (10).
Natural History
The natural history of cervical cancer has been studied extensively, and persistent infection of the cervix with certain high-risk types of HPV has been well established as a necessary cause of cervical cancer. HPV is a very common sexually transmitted infection (STI). Most infections clear spontaneously within one to two years; those persistent high-risk types, may progress to cervical cancer precursors, and ultimately to invasive cervical cancer. High-risk types of HPV are identified in nearly all cancers of the cervix. Strong evidences suggests that HPV infection precedes the development of cervical cancer by decades (10–30 years) and that persistent infection with HPV is necessary for the development and progression of precancerous lesions of the cervix, either to higher grades of precancerous disease or to cancer. Therefore, it is rare for cervical cancer to develop in a woman less than 30 years of age (WHO 2006). This long precancerous stage provides an excellent opportunity for effective intervention measures (figure 4). There is little geographic variation in the predominant HPV types associated with cervical cancer. A worldwide study that evaluated worldwide genotype distribution HPVinfection in 10,575 histologically confirmed cases of invasive cancer from 38 countries in Asia, Europe, Latin America and the Caribbean, North America, Oceania, and Sub-Saharan Africa over a 60-year period found that 85% of the cases were positive for HPV DNA. HPV types 16, 18, and 45 were the three most common types in each histologic form of cervical cancer (squamous cell, adenocarcinoma, and Adenosquamous carcinoma), accounting for 61%, 10%, and 6%, respectively (1, 12-14).


HPV E6 and E7 genes encode multifunctional proteins that bind primarily to cellular p53 and pRB proteins, disrupt their functions, and alter cell cycle regulatory pathways, leading to cellular transformation (14, 17).
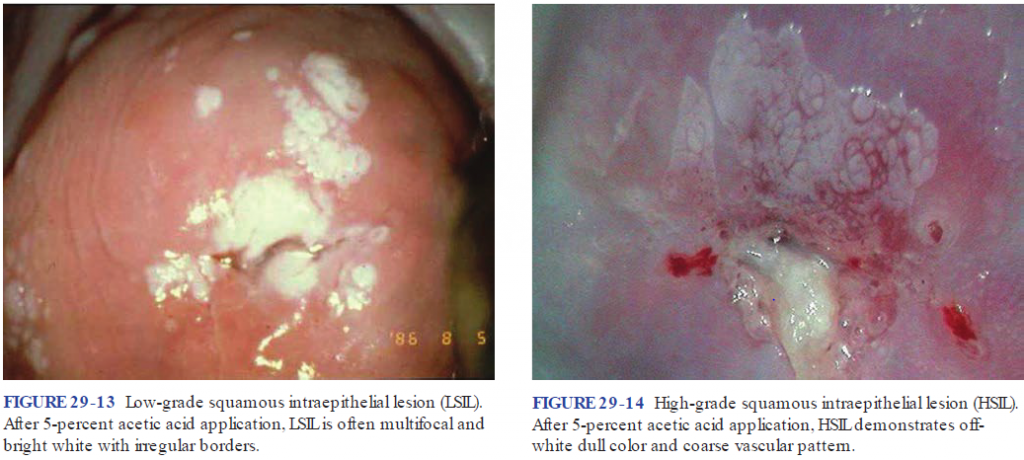
Description of the spectrum of squamous and glandular abnormalities seen in cervical cytology and histology using common and easy terminology is important. In many parts of the world, including Europe, the Bethesda System is used which distinguishes low-grade squamous intraepithelial lesion (LSIL) from high-grade squamous intraepithelial lesion (HSIL) and cancer and describes separate ‘equivocal’ categories of atypical squamous and glandular cells for instances where a clear distinction between benign and precancerous changes cannot be made (table 1). Follow-up studies and molecular analysis showed that LSIL is closely related to reversible HPV infection and is usually (but not always) associated with episomal HPV rather than virus integrated into the host genome. This means that LSIL may logically be managed as a potentially reversible lesion albeit with a risk of progression to HSIL. Although CIN2 has a greater likelihood of regression than CIN3, managing it as a condition at risk for progression is warranted. HSIL is regarded as a condition with a sufficiently high risk of invasion to warrant immediate investigation and ablation or excision (15).

Clinical Presentation, Diagnosis, Staging, and Treatment Options
A Six-Year Study of the Clinical Presentation of Cervical Cancer at a State Teaching Hospital in Southeast Nigeria reported that the major presenting complaints were abnormal vaginal bleeding (postcoital, irregular, or postmenopausal) (86.9%), offensive vaginal discharge (41%), and weight loss, pain (23%), Constipation (8.2%), Hematuria (4.9%) and leg swelling (3.3%). Around 40% patients had stage III disease and 17% stage IV at presentation (16). Diagnosis is based on History, physical examination, baseline investigations (e.g., CBC, RFT, LFT) and imaging studies (U/S, CT/MRI, CXR). But, histologic examination (from punch biopsy) is needed for confirmation of the diagnosis and to differentiate the histologic type of cancer. Cervical cancer is staged clinically, for example, through a pelvirectal examination and speculum examination combined with the above investigations. TNM classification and the International Federation of Gynecology and Obstetrics (FIGO) staging are used for staging (figure 5). Treatment of cervical cancer is determined by the stage of the disease at presentation (table 2). Options include surgery, Radiotherapy, Chemoradiotherapy, chemotherapy and palliative care. Only few women in LMICs have access to better cancer care due to both infrastructure and inadequate human power (e.g. only 23 African countries have radiotherapy services; in Ethiopia, there are only 13 oncologists and one radiotherapy center present at the moment) (1, 13, 16-18).

Prevention of Cervical Cancer
Secondary Prevention
Secondary prevention aims to prevent invasive cervical cancer by detecting and treating precancerous lesions of the cervix before they progress to cancer. The effectiveness of screening is evaluated by the reduction in cervical cancer incidence and mortality observed following screening. Historically, cervical cancer screening was based on examining cells collected from the surface of the cervix by Pap smear (cytology), followed by colposcopy for women with abnormal smears and histological assessment, followed by surgical treatment for histologically proven cancer precursors. This approach resulted in dramatic reductions in cervical cancer incidence and mortality. However, in LMICs, cytology-based screening programs and/or DNA typing of HPV are usually beyond the capacity of many health services. These include highly trained personnel, well equipped laboratories, transport of specimens, and an effective system for collecting information and following up patients. In addition, the demands of other competing health needs often result in a lack of resources or political will to make cervical cancer screening a priority. Consequently, several new tools have been developed that are better suited to these settingsincluding Visual inspection of the cervix using acetic acid (VIA) or Lugol’s iodine (VILI) which allows identification of pre-cancerous lesions in the clinic instead of the laboratory. With adequate training, any health care provider, including doctors, primary care staff (nurses, midwives) can effectively perform the procedure. VIA involves applying a 3–5% acetic acid solution to the cervix and then examining it with the naked eye using a bright light source figure 6). No expensive equipment or supplies are needed, and screening takes less than five minutes. A well-defined aceto-white area close to the transformation zone indicates a positive test. VIA provides an immediate result that can be used to decide on treatment on the same day (“screen and treat”), usually with cryotherapy, which requires training but no surgery or anesthesia. VIA may perform as well as or better than cervical cytology in identifying pre-cancerous lesions. VIA sensitivity and specificity are variable as they are highly dependent on the training and skill care giver. Nevertheless, different studies have demonstrated that using VIA correctly identified between 45% and 79% of women at high risk of developing cervical cancer. In comparison, the sensitivity of cytology has been shown to be between 47 and 62%. Paired with cryotherapy, VIA has successfully been implemented as a relatively simple, acceptable, and inexpensive method of treating cervical lesions and preventing development of cervical cancer in resource-limited settings. In our country, the 2015 national guideline for cervical cancer prevention and control recommend cytology based screening for large scale screening programs, if sufficient resource available; Visual screening methods (VIA and VILI) in resource limited but closely monitored settings, and HPV DNA tests as primary screening methods, only in pilot projects or other closely monitored settings. They can be used in conjunction with cytological or other tests where sufficient resources exist, and it should not be used before 30 years of age (13). The accuracy of the test decreases with the increasing age and cannot be used in post menopausal women. Moreover, their ultimate impact on cervical cancer incidence and mortality will not be known until large ongoing population-based studies are completed (table 2). Treatments of the precancerous lesions include ablative techniques (cryotherapy and cold coagulation), excisional technique (loop electrocautery excision procedure (LEEP)) and cone biopsy (1, 11, 13).
Though simple and suitable for setups in LMIC, performance of VIA is not without problems. . These difficulties include inadequate coverage, lost from follow-up of the large number of women needing treatment. This was evidenced from a study on the WHO sponsored project in six Sub-Saharan African countries (Madagascar, Malawi, Nigeria, Tanzania, Uganda, and Zambia) from 2005 through 2009. In all, 19,579 women were screened with VIA. Of these, 11.5% were VIA-positive; cancer was suspected in 1.7%. Of the VIA-positive women, 87.7% were eligible for cryotherapy, but only 60.9% were treated, and 34.6% were lost to follow-up. Of the women treated, only 39.1% were treated during the same visit as the screening. No information was available for 230 (70.5%) of the 326 women in whom cancer was suspected (16).

HPV Testing
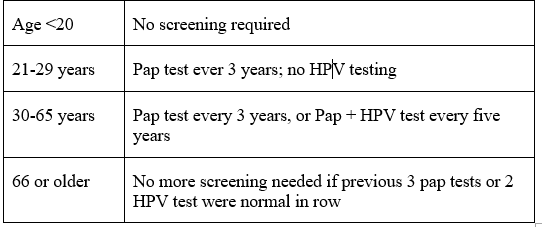
This are new screening procedures are based on the detection of high-risk HPV DNA in vaginal or cervical smears. A sample of cells is collected from the cervix or vagina using a swab or small brush by a health care provider or by the woman herself. Self collection, which can be done at home, is accepted by women and could significantly increase participation in screening, particularly by women who are reluctant to undergo a gynecological examination or who live in remote areas. Studies comparing the two collection methods have shown that self-collection is less sensitive than provider-collection. In either case, the specimen containers are transported to a laboratory where they are processed. Detection of high-risk HPV does not necessarily mean that precancers or cancer is present; it indicates simply that there is an HPV infection. As mentioned earlier, HPV infections are extremely common in women under 35 years, and most of them resolve spontaneously. When detection of HPV is used as a primary screening test, the sensitivity for detection of precancers and cancer ranges from 50% to 95%, with most studies reporting high sensitivity of 85% or more. The specificity ranges from 50% to 95%, with an average of 84%. In women aged 35 years or older, HPV DNA tests perform better because in these women a positive test is more likely to be due to a persistent infection than younger women. The average sensitivity and specificity in this age group are 89% and 90% respectively. The combination of cytology and HPV testing has very high sensitivity and negative predictive values approaching 100%. The high negative predictive value of HPV testing (nearly 100%) allows the extension of the screening interval, with consequent savings that can offset the possibly higher cost of the test compared with cytology. Screening with HPV testing under age 30 is not recommended, as HPV infection in this group of women is common, and most infections are likely to be transient with a low likelihood of developing into cancer. HPV DNA tests currently require sophisticated and expensive laboratory equipment, and reliable methods of transport present major challenges, and the feasibility of HPV testing has not been demonstrated in low-resource settings. Efforts are under way to develop new, faster, highly sensitive and less costly test for HPV is under development but is not yet available.

Therefore, HPV use as the primary screening test is not universal. In many countries including the USA, primary HPV testing has been recommended only in combination with cytology to improve the sensitivity of the screening or as a triage tool to assess which women with borderline Pap results need to be referred for colposcopy. The main indication is a Pap result of ASC-US, who test positive for high risk HPV will need to be referred for colposcopy, biopsy and treatment of visible lesions, reducing significantly the number of colposcopies (1, 13).
The U.S. Food and Drug Administration (FDA) has approved five of the many tests available for routine laboratory service (table 3); other tests that use PCR technology are being used in many clinical studies (1).
Primary Prevention
Primary prevention involves prevention of infection with HPV which is transmitted through sexual contact. Primary prevention can be achieved through behavioral change changes (abstinence, consistent condom use, limiting sexual partners) and the use of biological methods (HPV vaccination) which are a major breakthrough in preventing cervical cancer. Condoms offer only a partial protection against HPV transmission, because the virus can exist on body surfaces not covered by the condom. Currently, there are two types of HPV vaccines: the bivalent vaccine (Cervarix) and quadrivalent vaccine (Gardasil). These have been evaluated in large, randomized, placebo-controlled clinical trials and proven to be safe, immunogenic, and highly efficacious at preventing HPV infection for up to 8-10 years after vaccination. These vaccines prevent both persistent cervical infection with the types included in the vaccines in women not previously exposed to HPV infection, as well as Preinvasive lesions of the anogenital tract associated with the types present in the vaccines in males and females. In addition, the quadrivalent vaccine prevents genital warts caused by HPV types 6 and 11. Bivalent and quadrivalent vaccines appear to offer full protection against types 16 and 18 and some evidence suggests that the immune response to vaccination against types 16 and 18 also provides some cross-protection against types 45 and 31, which are important in the etiology of cervical cancer, thereby increasing the projected protection from vaccination to 75–80%. Both vaccines are given in a series of three 0.5 ml intramuscular (IM) injections within six months, and require storage and transport in a cold chain system (table 5). Women with past or current HPV infection are not candidates for vaccination. It would be premature to consider adding an HPV vaccine to infant EPI as no completed studies have included infants though several studies including young children are ongoing (1,13).

In an open label noninferiority, immunogenicity trial conducted in 15 countries and involving more than 1500 adolescent girls and boys aged 9-14years and young adults aged 16-26, two doses of 9 valent HPV vaccine administered at 6 or 12 months apart or the three doses administered over 6 month. The primary end point was antibody response each HPV type after a month of the last dose. It was found that the 9vHPV vaccine was noninferior to a 3 dose regimen in these cohorts of adolescents and young adults. 9vHPV targets HPV 6,11,16,18, 31,33,45,52, and 58 (22). Based on these results, FDA approved 9vHPVfor use in a 2 doses regimen for girls and boys aged 9-14.
Challenges to Implementing HPV Vaccination
The most obvious challenge is cost. The current price of both bivalent and quadrivalent vaccines is high, although the costs have decreased considerably as a result of initiatives. HPV is the most common STI in the world. Ideally, the vaccine should be administered to girls and possibly boys prior to the onset of sexual activity, the age of which varies considerably by country and culture. Unlike vaccinating infants and children, establishment of systematic approach (e.g., school based, health facility based, outreach or a combination) is needed for these target groups. This needs a great deal of political will. Moreover, both vaccines require a cold chain and thus a reliable source of electricity, which is absent in many LMICs, particularly in Sub-Saharan Africa. The need for three injections and follow-up poses its own challenges. However, as discussed above, that the immunogenicity and efficacy of two doses of the vaccine may be comparable to three doses, a promising development that could simplify the logistics and reduce the cost of HPV vaccination programs. The effect of vaccine on the incidence of cervical cancer would not be noticeable for some decades after introduction of the HPV vaccine. Therefore, widespread screening for cervical cancer needs to continue, even after an HPV vaccine program is fully implemented, in order to detect cervical abnormalities in the unvaccinated, previously infected population, and cancer caused by other HPV serotypes (1, 13).
Based on the burden of cervical cancer and recommendations from WHO for resource-poor countries (high-quality screening is not widespread; vaccination coverage is high can be maintained at >70%, and the cost of a three-dose course is low (< US$10-25)), Ethiopia launched HPV vaccination on 3rd December 2018. It was initially planned to introduce the vaccine through the routine immunization program for about 6 million girls of 9-14years of age. However, due to a global HPV vaccine shortage, the country introduced the vaccine in a single age cohort (14 years old girls) in the first year, and hopes to expand the service. The vaccine is primarily delivered through school based approach in both public and private schools. Out of school girls will be able to access the vaccine at any health facility in all nine regional states and two city administrations (13, 19).
Conclusion
Cervical cancer remains one of the most common cancers among women living in LMICs, yet it is a preventable and treatable cancer. Screening and vaccination are shown to be highly cost-effective public health interventions. Resource-constrained countries have been unable to initiate or sustain cytology-based cervical cancer screening programs because of weak health care infrastructure and prohibitive cost. There are two new avenues for cervical cancer prevention:
- Primary prevention through prophylactic vaccination (of preadolescent girls) against the most common HPV types causally associated with cervical cancer.
- Use of alternative screening tests and strategies for cervical cancer prevention, namely, VIA/VILI and HPV DNA testing. Both tests have their pros and cons, but the development of a highly reproducible, reliable, and accurate point-of-care HPV DNA test (or an alternative test yet to be developed fulfilling these criteria) will enable women to be screened and treated in one visit and without the need for colposcopy and laboratory infrastructure.
Dr. Abdu Adem
Assistant professor of Oncology
References
- GELBAND, H., et al. Summary–Cancer: Disease Control Priorities, (Volume 3). 2015.
- https://www.webmd.com/cancer/cervical-cancer/cervical-cancer(Accessed on 25/9/2019)
- https://www.mayoclinic.org/diseases-conditions/cervical-cancer/symptoms-causes/syc-20352501 (Accessed on 25/9/2019)
- https://www.cdc.gov/cancer/cervical/basic_info/index.htm (Accessed on 2592019)
- BRAY, Freddie, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians, 2018, 68.6: 394-424.
- ARBYN, Marc, et al. Worldwide burden of cervical cancer in 2008. Annals of oncology, 2011, 22.12: 2675-2686.
- TIMOTEWOS, Genebo, et al. First data from a population based cancer registry in Ethiopia. Cancer epidemiology, 2018, 53: 93-98.
- LI, Xiao, et al. Systematic literature review of risk factors for cervical cancer in the Chinese population. Women’s Health, 2018, 14: 1745506518816599.
- BENNETT, John E.; DOLIN, Raphael; BLASER, Martin J. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases: 2-Volume Set. Elsevier Health Sciences, 2014.
- WORLD HEALTH ORGANIZATION. REPRODUCTIVE HEALTH, et al. Comprehensive cervical cancer control: a guide to essential practice. World Health Organization, 2006.
- MAKUZA, Jean Damascène, et al. Prevalence and risk factors for cervical cancer and pre-cancerous lesions in Rwanda. Pan African Medical Journal, 2015, 22.1.
- DE SANJOSE, Silvia, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. The lancet oncology, 2010, 11.11: 1048-1056.
- Federal Democratic Republic of Ethiopia Ministry of Health Guideline for Cervical Cancer Prevention and Control in Ethiopia, January 2015.
- Burd EM. Human papillomavirus and cervical cancer. ClinMicrobiol Rev. 2003 Jan;16(1):1-17. doi: 10.1128/cmr.16.1.1-17.2003. PMID: 12525422; PMCID: PMC145302.
- https://www.eurocytology.eu/en/course/1144 (Accessed on 27/9/2019)
- EZE, Justus N.; EMEKA-IREM, Esther N.; EDEGBE, Felix O. A six-year study of the clinical presentation of cervical cancer and the management challenges encountered at a state teaching hospital in southeast Nigeria. Clinical Medicine Insights: Oncology, 2013, 7: CMO. S12017.
- Williams Gynecology, 3rd edition, 2016.
- BHATLA, Neerja, et al. Revised FIGO staging for carcinoma of the cervix uteri. International Journal of Gynecology & Obstetrics, 2019, 145.1: 129-135.
- afro.who.int.news/ehiopia (accessed on 29/9/2019)
- usupreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening (accessed on 29/9/2019)
- Center for Disease Control and Prevetion
- Iverson O-E,Miranda MJ,Ulied A, et al, immunogenicity of 9 valent HPV vaccine using 2 dose regimens in girls and boys vs 3 dose regimen in women. JAMA 2016;316:2411-21